Bimomed Amber Material Details

General Information
BioMed Amber Resin is a specialized photopolymer resin formulated for Stereolithography 3D printing (SLA) with Formlabs printers. This unique resin offers a range of properties suited for various medical and biotechnological applications. BioMed Amber Resin is renowned for its high strength, biocompatibility, and detailed reproduction capabilities. However, it's essential to consider some of its unique characteristics and limitations.
Sterilization and Disinfection Compatibility
BioMed Amber Resin is designed to meet the stringent requirements of medical applications and offers compatibility with various sterilization and disinfection methods:
- Electron Beam Irradiation: BioMed Amber Resin can withstand electron beam irradiation at 35 kGy.
- Ethylene Oxide: It is resistant to 100% ethylene oxide at 55°C for 180 minutes.
- Gamma Radiation: BioMed Amber Resin can endure gamma radiation in the range of 29.4 to 31.2 kGy.
- Steam Sterilization: It is suitable for steam sterilization in an autoclave at 134°C for 20 minutes or at 121°C for 30 minutes.
Chemical Disinfection Compatibility
BioMed Amber Resin can undergo chemical disinfection using 70% isopropyl alcohol for 5 minutes, ensuring effective and rapid disinfection.
Compliance with ISO Standards
This resin has been meticulously developed in accordance with the following ISO standards:
- EN ISO 13485:2016: Requirements for Quality Management Systems in Medical Devices.
- EN ISO 14971:2012: Application of Risk Management to Medical Devices.
BioMed Amber Resin Material Highlights:
- High Tensile Strength: With a tensile strength of 73 MPa (ASTM D638-10 Type IV), BioMed Amber Resin provides exceptional mechanical performance.
- Robust Young's Modulus: Exhibiting a Young's modulus of 2900 MPa (ASTM D638-10 Type IV), it offers high stiffness and structural integrity.
- Elongation Capability: BioMed Amber Resin maintains an elongation of 12% (ASTM D638-10 Type IV), ensuring flexibility where required.
- Durable Flexural Strength: With a flexural strength of 103 MPa (ASTM D790-15 Method B), it provides durability in bending applications.
- Stiff Flexural Modulus: Its flexural modulus measures at 2500 MPa (ASTM D790-15 Method B), ensuring structural stability.
- Tough Shore D Hardness: BioMed Amber Resin exhibits a Shore D hardness of 67D (ASTM D2240-15 Type D), combining strength with toughness.
- Impact Resilience: It offers an Izod impact resistance of 28 J/m (ASTM D256-10 Method A) and an unnotched Izod impact resistance of 142 J/m (ASTM D4812-11), making it resilient against sudden loads.
- Impressive Thermal Performance: BioMed Amber Resin boasts a heat deflection temperature under load of 65°C at 1.8 MPa and 78°C at 0.45 MPa (ASTM D648-18 Method B), ensuring stability in elevated temperature environments.
- Thermal Expansion: Its coefficient of thermal expansion is 66 μm/m/°C (ASTM E831-14), contributing to dimensional stability.
Printing in BIOMED Amber
Minimum Wall: 1 mm
Smalest Detail : 0.3
Layer hight : 0.2 mm
Max Print size: 256 x 256 x 256 mm
Tollerance: ± 0,2 mm
Delivery Times: typicaly 2-3 Businessdays

Pro`s and Con`s
Pro:
- Exceptional Clarity: BioMed Amber Resin produces parts with outstanding transparency and optical clarity, ideal for medical visualization models.
- Biocompatible: The resin is biocompatible, making it suitable for a wide range of medical and dental applications, including surgical guides and orthodontic models.
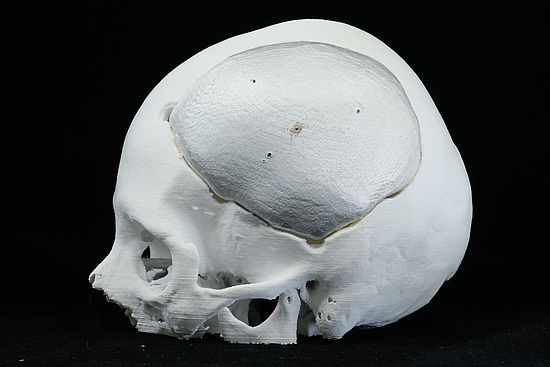
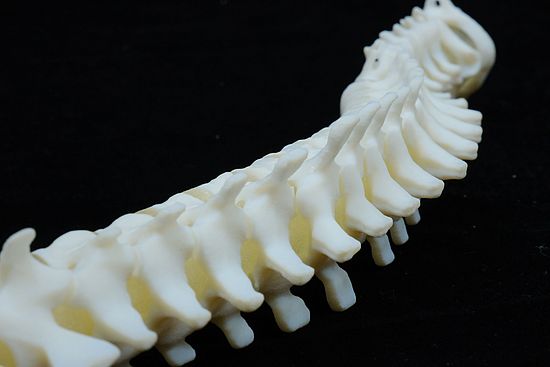
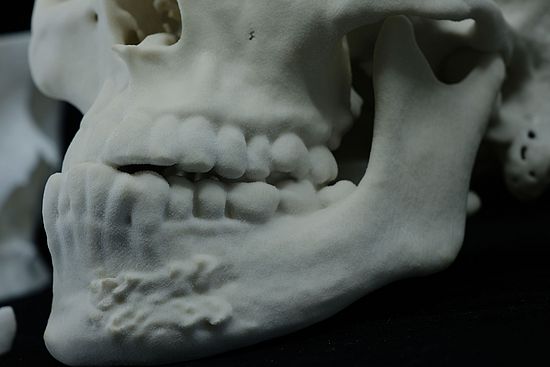
- Fine Detail Reproduction: BioMed Amber Resin can accurately reproduce fine details, making it valuable for creating anatomical models and dental impressions.
- Smooth Surface Finish: Parts printed with BioMed Amber Resin often exhibit a smooth and polished surface finish, enhancing their appearance.
Con:
- UV Sensitivity: Prolonged UV exposure may impact the material's optical properties, so it's not recommended for outdoor use.
- Post-Processing Requirements: Achieving the highest optical clarity may necessitate additional post-processing steps, potentially increasing production time.
- Manual Support Removal: As with other SLA materials, BioMed Amber Resin may require manual support removal, which can be labor-intensive for complex parts.
Applications of BIOMED Amber 3D Print



BioMed Amber Resin is well-suited for various medical and biotechnological applications, thanks to its mechanical strength and biocompatibility. Some common use cases include:
- Medical Devices and Components: BioMed Amber Resin is suitable for creating various medical devices and their components, ensuring safety and compliance.
- Research and Development: Researchers can utilize this resin to prototype medical innovations and conduct experiments.
- Surgical Planning and Implant Measurement Tools: It aids in the development of tools for surgical planning and implant measurement.
The unique properties of BioMed Amber Resin make it an ideal choice for applications that demand both mechanical performance and biocompatibility.
Technical specifications
Mechanical Properties
Property | Test Method | Value |
Tensile Strength | ASTM D638-10 (Type IV) | 73 MPa |
Young's Modulus | ASTM D638-10 (Type IV) | 2900 MPa |
Elongation | ASTM D638-10 (Type IV) | 12% |
Flexural Strength | ASTM D790-15 (Method B) | 103 MPa |
Flexural Modulus | ASTM D790-15 (Method B) | 2500 MPa |
Shore D Hardness | ASTM D2240-15 (Type D) | 67 D |
Izod Impact Strength | ASTM D256-10 (Method A) | 28 J/m |
Izod Impact Strength (Unnotched) | ASTM D4812-11 | 142 J/m |
Heat Deflection Temperature (1.8 MPa) | ASTM D648-18 (Method B) | 65 °C |
Heat Deflection Temperature (0.45 MPa) | ASTM D648-18 (Method B) | 78 °C |
Coefficient of Thermal Expansion | ASTM E831-14 | 66 μm/m/°C |
Water Absorption | ASTM D570-98 (2018) | 0.54% |
Compatibility with Sterilization Methods
Sterilization Method | Details |
Electron Beam Irradiation | 35 kGy |
Ethylene Oxide | 100% ethylene oxide at 55°C for 180 minutes |
Gamma Irradiation | 29.4 – 31.2 kGy |
Steam Sterilization | Autoclave at 134°C for 20 minutes |
Steam Sterilization | Autoclave at 121°C for 30 minutes |
BioMed Amber Resin Biocompatibility
BioMed Amber Resin-printed specimens have been thoroughly assessed to meet stringent biocompatibility standards, as follows:
- ISO 10993-5:2009 Non-Cytotoxic
- ISO 10993-3:2014 Non-Mutagenic
- ISO 10993-10:2010/(R)2014 Non-Irritant
- ISO 18562-2:2017 Non-Particulate Shedding
- ISO 10993-10:2010/(R)2014 Non-Sensitizing
- ISO 18562-3:2017 No VOC Emissions
- ISO 10993-17:2002, ISO 10993-18:2005 Non-Toxic (Subacute/Subchronic)
- ISO 10993-11:2017 No Evidence of Acute Systemic Toxicity
- ISO 10993-11:2017/USP, General Chapter <151>, Pyrogen Test Non-Pyrogenic
Compatibility with Disinfectants
Isopropyl Alcohol (70%) | 5 minutes |
Compliance with ISO Standards
The development of this resin aligns with the following ISO standards:
- EN ISO 13485:2016 Medical Devices - Quality Management Systems - Regulatory Purposes
- EN ISO 14971:2012 Medical Devices - Application of Risk Management to Medical Devices