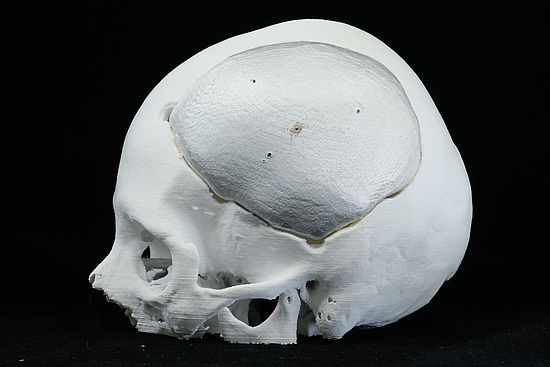
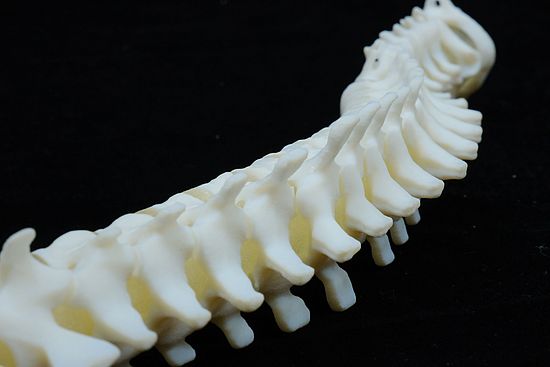
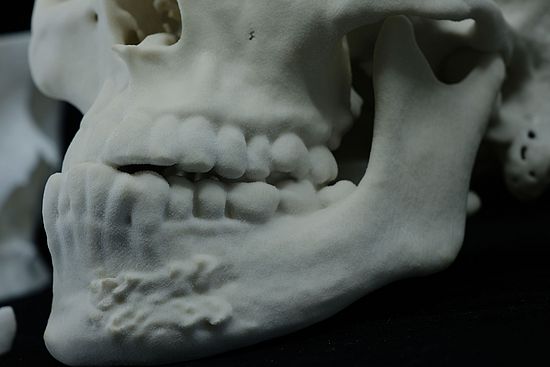
Biomed Clear Material Details
General Information
BioMed Amber Resin is a specialized photopolymer resin formulated for Stereolithography 3D printing (SLA) with Formlabs printers. This unique resin offers a range of properties suited for various medical and biotechnological applications. BioMed Amber Resin is renowned for its high strength, biocompatibility, and detailed reproduction capabilities. However, it's essential to consider some of its unique characteristics and limitations.
Sterilization and Disinfection Compatibility
BioMed Amber Resin is designed to meet the stringent requirements of medical applications and offers compatibility with various sterilization and disinfection methods:
- Electron Beam Irradiation: BioMed Amber Resin can withstand electron beam irradiation at 35 kGy.
- Ethylene Oxide: It is resistant to 100% ethylene oxide at 55°C for 180 minutes.
- Gamma Radiation: BioMed Amber Resin can endure gamma radiation in the range of 29.4 to 31.2 kGy.
- Steam Sterilization: It is suitable for steam sterilization in an autoclave at 134°C for 20 minutes or at 121°C for 30 minutes.
Chemical Disinfection Compatibility
BioMed Amber Resin can undergo chemical disinfection using 70% isopropyl alcohol for 5 minutes, ensuring effective and rapid disinfection.
Compliance with ISO Standards
This resin has been meticulously developed in accordance with the following ISO standards:
- EN ISO 13485:2016: Requirements for Quality Management Systems in Medical Devices.
- EN ISO 14971:2012: Application of Risk Management to Medical Devices.
BioMed Amber Resin Material Highlights:
- High Tensile Strength: With a tensile strength of 73 MPa (ASTM D638-10 Type IV), BioMed Amber Resin provides exceptional mechanical performance.
- Robust Young's Modulus: Exhibiting a Young's modulus of 2900 MPa (ASTM D638-10 Type IV), it offers high stiffness and structural integrity.
- Elongation Capability: BioMed Amber Resin maintains an elongation of 12% (ASTM D638-10 Type IV), ensuring flexibility where required.
- Durable Flexural Strength: With a flexural strength of 103 MPa (ASTM D790-15 Method B), it provides durability in bending applications.
- Stiff Flexural Modulus: Its flexural modulus measures at 2500 MPa (ASTM D790-15 Method B), ensuring structural stability.
- Tough Shore D Hardness: BioMed Amber Resin exhibits a Shore D hardness of 67D (ASTM D2240-15 Type D), combining strength with toughness.
- Impact Resilience: It offers an Izod impact resistance of 28 J/m (ASTM D256-10 Method A) and an unnotched Izod impact resistance of 142 J/m (ASTM D4812-11), making it resilient against sudden loads.
- Impressive Thermal Performance: BioMed Amber Resin boasts a heat deflection temperature under load of 65°C at 1.8 MPa and 78°C at 0.45 MPa (ASTM D648-18 Method B), ensuring stability in elevated temperature environments.
- Thermal Expansion: Its coefficient of thermal expansion is 66 μm/m/°C (ASTM E831-14), contributing to dimensional stability.
Printing in BIOMED Clear
Minimum Wall: 0.3 mm
Smalest Detail: 0.05 mm
Layer hight: 0.05 mm
Max Print size:145 × 145 × 185 mm
Tollerance: 0.2% min ±0.15 mm
Delivery Times: Typicaly 5-8 Businessdays

Pro`s and Con`s
Pro:
- Exceptional Clarity: Biomed Clear produces parts with outstanding transparency and optical clarity, ideal for medical visualization models.
- Biocompatible: The resin is biocompatible, making it suitable for a wide range of medical and dental applications, including surgical guides and orthodontic models.
- Fine Detail Reproduction: Biomed Clear can accurately reproduce fine details, making it valuable for creating anatomical models and dental impressions.
- Smooth Surface Finish: Parts printed with Biomed Clear often exhibit a smooth and polished surface finish, enhancing their appearance.
Con:
- UV Sensitivity: Prolonged UV exposure may impact the material's optical properties, so it's not recommended for outdoor use.
- Post-Processing Requirements: Achieving the highest optical clarity may necessitate additional post-processing steps, potentially increasing production time.
- Manual Support Removal: As with other SLA materials, Biomed Clear may require manual support removal, which can be labor-intensive for complex parts.
Applications of BIOMED Clear 3D Print



Biomed Clear resin is well-suited for various medical and biotechnological applications, thanks to its clarity and biocompatibility. Some common use cases include:
- Medical Visualization Models: Biomed Clear is ideal for creating anatomical models and transparent medical visualization aids for educational and training purposes.
- Dental Models: It is commonly used in dentistry for producing dental impressions, orthodontic models, and clear aligners.
- Surgical Guides: Biomed Clear can be used to fabricate precise surgical guides for dental and medical procedures.
- Biotechnological Prototypes: Researchers and biotechnologists often use this resin to create prototypes for various lab equipment and devices.
- Transparent Prototypes: Any application requiring clear or translucent prototypes can benefit from Biomed Clear's optical clarity.
Technical specifications
Mechanical Properties
Property | Test Method | Value |
Tensile Strength | ASTM D638-10 (Type IV) | 52 MPa |
Young's Modulus | ASTM D638-10 (Type IV) | 2080 MPa |
Elongation | ASTM D638-10 (Type IV) | 12% |
Flexural Strength | ASTM D790-15 (Method B) | 84 MPa |
Flexural Modulus | ASTM D790-15 (Method B) | 2300 MPa |
Shore D Hardness | ASTM D2240-15 (Type D) | 78D |
Izod Impact Strength | ASTM D256-10 (Method A) | 35 J/m |
Izod Impact Strength (Unnotched) | ASTM D4812-11 | 449 J/m |
Heat Deflection Temperature (1.8 MPa) | ASTM D648-18 (Method B) | 54 °C |
Heat Deflection Temperature (0.45 MPa) | ASTM D648-18 (Method B) | 67 °C |
Coefficient of Thermal Expansion | ASTM E831-14 | 82 μm/m/°C |
Water Absorption | ASTM D570-98 (2018) | 0.54% |
Compatibility with Sterilization Methods
Sterilization Method | Details |
Electron Beam Irradiation | 35 kGy |
Ethylene Oxide | 100% ethylene oxide at 55 °C for 180 minutes |
Gamma Irradiation | 29.4 – 31.2 kGy |
Steam Sterilization | Autoclave at 134 °C for 20 minutes |
Steam Sterilization | Autoclave at 121 °C for 30 minutes |
Compatibility with Disinfectants
Disinfectant | Details |
Isopropyl Alcohol (70%) | 5 minutes |
BioMed Clear Resin-printed specimens have been evaluated according to ISO 10993-1:2018, ISO 7405:2018, and ISO 18562-1:2017 standards and meet the requirements associated with the following biocompatibility risks:
- ISO 10993-5:2009 Non-Cytotoxic
- ISO 10993-3:2014 Non-Mutagenic
- ISO 10993-10:2010/(R)2014 Non-Irritant
- ISO 18562-2:2017 Non-Particulate Shedding
- ISO 10993-10:2010/(R)2014 Non-Sensitizing
- ISO 18562-3:2017 No VOC Emissions
- ISO 10993-17:2002, ISO 10993-18:2005 Non-Toxic (Subacute/Subchronic)
- ISO 10993-11:2017 No Evidence of Acute Systemic Toxicity
- ISO 10993-11:2017/USP, General Chapter <151>, Pyrogen Test Non-Pyrogenic
The resin has been developed in accordance with the following ISO standards:
- EN ISO 13485:2016 Medical Devices - Quality Management Systems - Regulatory Purposes
- EN ISO 14971:2012 Medical Devices - Application of Risk Management to Medical Devices